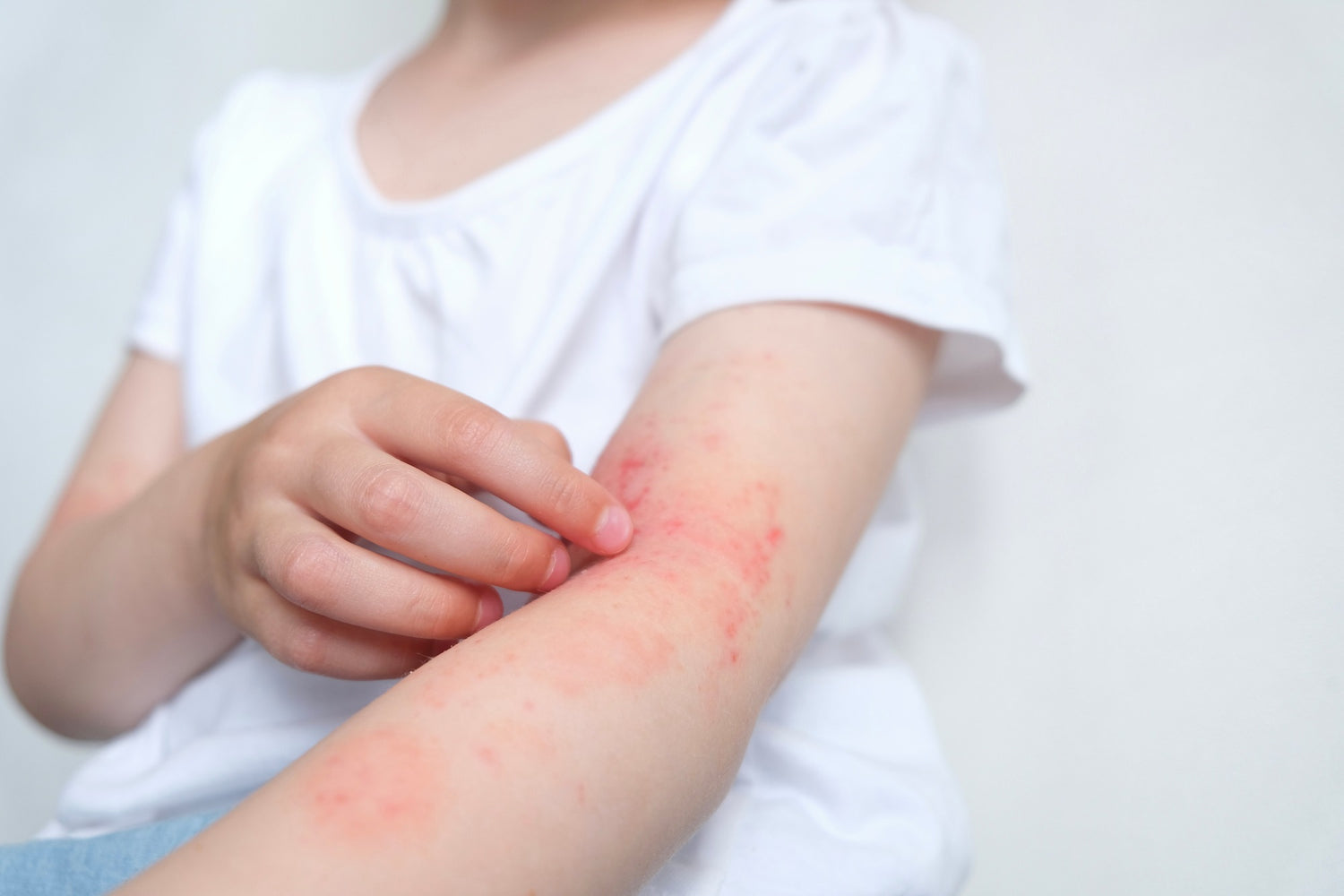
Eczema, or atopic dermatitis (AD), is a non-contagious, chronic, inflammatory skin disease (1). According to the National Eczema Society (2), eczema affects approximately one in five children in the UK and is characterised by dry, itchy skin that can disrupt sleep and daily activities (1, 3). It can also be linked with allergic conditions such as cow’s milk protein allergy (CMPA) (4).
Emerging research highlights a potential link between gut health and skin conditions. For example, eczema is often linked to an altered gut microbiome. As a result, probiotics have been proposed as a novel adjunctive treatment for eczema (5, 6). This article will explore the gut-skin axis and how specific probiotic strains might influence eczema outcomes in paediatrics.
Gut-Skin Axis
Research suggests that the gut and skin are connected, called the "gut-skin axis." This connection is facilitated by the modulation of the immune environment through the gut microbiome. The gut microbiome produces metabolites, neurotransmitters and hormones that can enter the bloodstream and influence the skin (7), such as anti-inflammatory cytokine IL-10 and decreased serum levels of the pro-inflammatory IL-17.
Disturbances in the normal gut microbiome, often called gut dysbiosis, have been observed in children with eczema (8-10). As a result, scientists now propose that the gut microbiome could be a potential target for managing eczema through probiotic interventions.
The Mechanisms of Actions
The exact mechanisms of how the gut microbiome influences the skin have not been fully established. However, it is theorised that a compromised skin barrier may be the first step in the development of eczema, which can lead to further skin inflammation and allergic sensitisation. It is suggested that type 2 cytokines, as well as IL-17 and IL-22, contribute to skin barrier dysfunction and the development of eczema (11).
A recent systematic review by Xue et al (11) noted that both single-strain and multi-strain probiotics positively affected eczema outcomes as measured by the SCORAD index. However, the authors emphasised that future long-term studies with larger sample sizes are required to fully understand these mechanisms and confirm the efficacy of specific probiotics in treating eczema.
It's also important to consider that the skin hosts its very own complex microbiome; therefore, optimal skin health will depend on cooperative interaction between the gut and skin microbiomes (12).
Current Treatment of Eczema
Standard Treatment for eczema
There is currently no cure for eczema as the causes can be multifactorial. In infants and older children not affected by CMPA, the primary treatments include emollients and topical steroids. They are essential for controlling eczema flares or managing severe symptoms and can provide much-needed relief for itching and preventing further skin damage.
Complimentary and Alternative Treatments for Eczema
For parents seeking complementary and alternative treatments, a systematic review showed that symptoms associated with eczema in adults were effectively managed using tea tree essential oils blended with sunflower seed or coconut oils.
The effectiveness of these was attributed to an antibacterial and anti-inflammatory property of the oils (13, 14). Coconut oil is useful for addressing skin dryness and itching (15).
Interestingly, honey also showed improvements in eczema symptoms such as erythema, oedema/papulation and excoriation. Honey led to marked improvement of severity and symptoms in 80% of patients in the treatment group and allowed reduction of topical corticosteroid use in the control group (16).
Finally, topical curcumin formulations including a microemulsion, a gel, and an ointment containing turmeric were efficacious at healing symptoms of erythema and oedema whereas, gels and ointments were more efficacious for pruritus and lichenification improvement (17).
While traditional treatments focus on symptom management, emerging research suggests a more proactive approach might lie in the gut.
Managing Infants with CMPA
Finally, for infants diagnosed with severe eczema and CMPA (4), treating the allergy itself can significantly improve and sufficiently address the infant’s eczema.
In this scenario, the recommended treatment is a cow’s milk protein-free diet trial, which involves switching from standard infant formula to an extensively hydrolysed formula. For more information, see article one in this 2-part series.
Probiotics in Eczema
The research on the effectiveness of probiotics on eczema has been conflicting as shown in systematic reviews (11, 18). For this reason, most guidelines, including those from the European Society of Allergy and Clinical Immunology and the European Society of Paediatric Gastroenterology, Hepatology and Nutrition, currently do not recommend probiotic supplementation for treating eczema (19).
However, the World Allergy Organization recommends probiotics for high‐risk infants because of their potential benefits for pregnant, breastfeeding women and infants to prevent AD (20). A notable example is Lactobacillus rhamnosus HN001.
Lactobacillus rhamnosus HN001
A randomised control study from New Zealand (21) explored the impact of administering HN001 from 35 weeks of pregnancy until 6 months post-partum, or until the end of breastfeeding if earlier. The study found that its protective effects against eczema extended up to at least 4 years of age. It also appeared to protect against rhinoconjunctivitis, suggesting its potential as a preventative treatment for infants at high risk of allergies.
What’s exciting is that in the follow-up study (22), when assessed at 11 years of age, the children from the HN001 group showed less than half the risk of eczema compared to children in the placebo group (p=0.015). Children taking HN001 also had a notable reduction in the risk of hay fever, asthma and rhinitis.
The authors suggest that HN001 may alter the developing immune system, suppressing the Th2‐mediated hypersensitivity response typical in AD and improving the skin barrier in genetically predisposed children.
These findings offer promising insights into the role of probiotics in managing eczema and AD, although more research is needed to understand if starting this probiotic after birth can yield similar benefits.
Lactobacillus rhamnosus GG
Lactobacillus rhamnosus GG is one of the most researched strains in children. However, its role in managing eczema in children shows mixed results. This is likely due to study variables such as probiotic dosage, family history, dietary and environmental factors.
Despite this, one randomised controlled trial by Schmidt et al in 2019 (23), found that children who received a combination of Lactobacillus rhamnosus GG and Bifidobacterium lactis BB‐12 starting at around 10 months of age had a lower incidence of eczema compared to those in the control group.
Additionally, the “Atopic March” study of 365 infants (24), revealed that using an extensively extensively hydrolysed formula containing rhamnosus GG for cow’s milk allergy management reduced the risk of developing atopic manifestations by 22% over 36 months.
In this study the probiotic group was compared to a rice hydrolysed formula cohort; a soy formula cohort; an extensively hydrolysed whey-based formula cohort; and an amino acid-based formula cohort. This study also noted that 81% of infants fed the probiotic-fortified casein-based formula returned to cow’s milk, versus only 42% of those fed a whey-based equivalent formula without probiotics (p<0.001).
Bifidobacterium Breve M-16V
A small randomised double-blind study (25) of 31 infants up to age 11 months with moderate to severe AD, examined the effects of an extensively hydrolysed whey-based formula fortified with or without prebiotics and Bifidobacterium breve M-16V at a dose of 1.0 × 108 cfu/g. After four months, all infants showed reduced severity of eczema. However, infants in the probiotic group experienced greater improvements.
This group also showed an increase in the inflammation marker CXCL9 and a change in chemokine ratios, suggesting a shift towards a less allergic and inflammatory state (potentially due to the pre and probiotics).
Similar results have been reported in another randomised trial of 90 infants with AD who received either an extensively hydrolysed formula with Breve M-16V or the same formula without (26). The authors concluded that SCORAD score improvement was significantly greater in the synbiotic than in the placebo group at week 12.
Additionally, a retrospective matched cohort study (27) showed that in infants with CMPA, treatment with an amino acid infant formula with Bifidobacterium Breve M-16V and prebiotics led to significantly fewer infants experiencing overall GI, skin, respiratory and/or ear infections than in the amino acid group without synbiotics (66% vs. 86% overall, p = 0.007).
Conclusion
This article explored the role of probiotics in the management of eczema, through modulation of the gut-skin axis. The strongest evidence supports starting probiotics early during pregnancy for high-risk infants and continuing their use until around 6 months of age.
However, once eczema is established, the effectiveness of probiotics shows mixed results. The best outcomes were observed when single-strain probiotics were used. Further research is essential to fully understand the role of probiotics in managing eczema, including determining the most effective strains and doses required for positive outcomes.
References
- Hadi HA, et al. 2021;11(9):936.
- National Eczema Society. Information and advice [Internet]. London: National Eczema Society; [cited 2024 Apr 29]. Available from: https://eczema.org/information-and-advice/
- Great Ormond Street Hospital for Children NHS Foundation Trust. Eczema [Internet]. Great Ormond Street Hospital; [date unknown] [cited 2024 Mar 20]. Available from: https://www.gosh.nhs.uk/conditions-and-treatments/conditions-we-treat/eczema/
- Fox A, et al. Clin Transl Allergy. 2019;9:40.
- Drago L, et al. Int J Immunopathol Pharmacol 2011;24(4):1037-48.
- Gore C, et al. Clin Exp Allergy. 2012;42(1):112-22.
- O'Neill CA, et al. Bioessays 2016;38(11):1167-76.
- Gore C, et al. J Allergy Clin Immunol 2008;121:135–40.
- Sepp E, et al. Clin Exp Allergy 2005;35:1141–6.
- Mah KW, et al. Int Arch Allergy Immunol 2006;140:157–63.
- Xue X, et al. Clin Transl Allergy 2023;13(7):e12283.
- Grice EA, et al. Science 2009;324(5931):1190–2.
- Deyno S, et al. Complement Ther Med 2019;47:102224.
- Jones VA, et al. JAAD Int. 2020;2:76-93.
- Diluvio L, et al. G Ital Dermatol Venereol 2019;154(1):32–6.
- Al-Waili NS. Complement Ther Med 2003;11(4):226–34.
- Khiljee S, et al. Pak J Pharm Sci 2015;28(6):2001–7.
- Pelucchi C, et al. Epidemiology 2012;23(3):402-14.
- Agache I, et al. Allergy 2021;76(4):988-1009.
- Fiocchi A, et al. Probiotics. World Allergy Organ J 2015;8(1):4.
- Wickens K, et al. Clin Exp Allergy 2012;42(7):1071-9.
- Wickens K, et al. Pediatr Allergy Immunol 2018;29(8):808-14.
- Schmidt RM, et al. Pediatr Allergy Immunol 2019;30(3):335-40.
- Nocerino R, et al. J Pediatr 2021;232:183-91.e3.
- Hulshof L, et al. Front Immunol. 2018;9:630.
- Van der Aa LB, et al. Clin Exp Allergy 2010;40:795-804.
- Sorensen K, et al. Nutrients. 2021;13(7):2205.